For US Healthcare Professionals only
PRESCRIBINGINFORMATION REQUEST
SAMPLES

NUZYRA is indicated for the treatment of Community-Acquired Bacterial Pneumonia (CABP) and Acute Bacterial Skin and Skin Structure Infections (ABSSSI) in adults caused by select susceptible microorganisms.

abscess, or wound infection treatment needs, consider
REAL-WORLD RESULTS
Cases for prescribing NUZYRA® empirically
These case studies reflect real patients using NUZYRA. The results presented are consistent with those observed in the OASIS-2 clinical trial in patients with ABSSSI.
Individual results may vary.
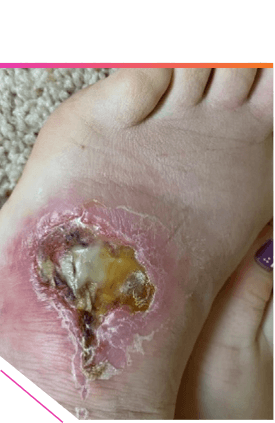
ADULT PATIENT
WITH SURGICAL
WOUND
DEHISCENCE
AND CELLULITIS1
- Hospital avoidance was important to the patient due to childcare concerns and limitations
- Treatment compliance was important in empiric treatment decision for an infection of this magnitude
INITIAL PRESENTATION1
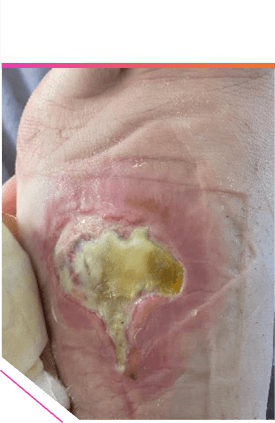
1 WEEK AFTER COURSE OF TREATMENT1
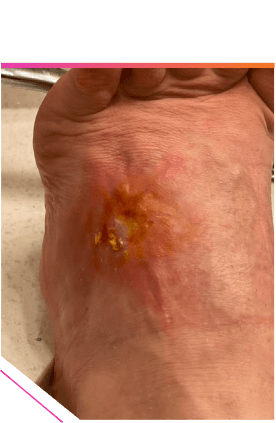
FINAL HEALED WOUND AFTER 3 MONTHS1
Terms of Use: This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0). The photos were taken by Dr. Philip Wrotslavsky and the original version can be found at https://www.fastracjournal.org/article/S2667-3967(23)00001-0/fulltext.
- Cellulitis surrounding large, dehiscent wound was clinically cured* in the outpatient setting with once-daily oral NUZYRA
Patient did not require admission or return to the hospital. After NUZYRA was started, the wound healed uneventfully over the next 6 weeks with local wound care, debridement, and negative pressure.
CLINICALLY CURED* WITH A
SINGLE COURSE OF TREATMENT1

*Clinical cure at the post-treatment evaluation was defined as survival after completion of study treatment without receiving any other antibacterial therapy or unplanned major surgical intervention, and having sufficient resolution of infection such that further antibacterial therapy is not needed.2

PATIENT PRESENTATION AND PAST MEDICAL HISTORY1,3
PMHx
- Patient is 35-year-old female; S/P surgical removal of ganglion cyst
- Anemia
- Anxiety
- Depression
- Fibromyalgia
- Hip arthroscopy
CURRENT MEDICATIONS
- Amphetamine/dextroamphetamine
- Fluoxetine
- Meloxicam
- Gabapentin
- Acetaminophen
LABORATORY FINDINGS
- WBC count 14.92 K/mm3
- Glucose WNL
SOCIAL HISTORY
- Single mother of 2 children
PHYSICAL FINDINGS
- Large ganglion cyst surgically removed from left foot
- Postoperative follow-up showed beginning of surgical wound dehiscence at 2 weeks
- Dehiscence had progressed at 4 weeks with increased pain and swelling
- At week 5, the patient developed signs of cellulitis with edema, erythema and increased pain
- No drainage
- No malodor
- Temperature 98°F
CLINICAL COURSE OF TREATMENT
- Initiated with NUZYRA oral loading dose 450 mg x 2 days, then 300 mg PO QD
- Total treatment duration was 14 days
PMHx=past medical history; PO=per os; QD=once a day; S/P=status post; WBC=white blood cell; WNL=within normal limits.
Safety data for NUZYRA
in the treatment of ABSSSI
Considering NUZYRA
for your patients?
View additional real-world
case study in ABSSSI
NUZYRA® (omadacycline) is a tetracycline-class antibacterial indicated for the treatment of adult patients with the following infections caused by susceptible microorganisms:
Community-Acquired Bacterial Pneumonia (CABP) caused by the following:
Streptococcus pneumoniae, Staphylococcus aureus (methicillin-susceptible isolates), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydophila pneumoniae.
Acute Bacterial Skin and Skin Structure Infections (ABSSSI) caused by the following:
Staphylococcus aureus (methicillin-susceptible and -resistant isolates), Staphylococcus lugdunensis, Streptococcus pyogenes, Streptococcus anginosus grp. (includes S. anginosus, S. intermedius, and S. constellatus), Enterococcus faecalis, Enterobacter cloacae, and Klebsiella pneumoniae.
USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of NUZYRA and other antibacterial drugs, NUZYRA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
NUZYRA is contraindicated in patients with known hypersensitivity to omadacycline or tetracycline-class antibacterial drugs, or to any of the excipients.
WARNINGS AND PRECAUTIONS
Mortality imbalance was observed in the CABP clinical trial with eight deaths (2%) occurring in patients treated with NUZYRA compared to four deaths (1%) in patients treated with moxifloxacin. The cause of the mortality imbalance has not been established. All deaths, in both treatment arms, occurred in patients > 65 years of age; most patients had multiple comorbidities. The causes of death varied and included worsening and/or complications of infection and underlying conditions. Closely monitor clinical response to therapy in CABP patients, particularly in those at higher risk for mortality.
The use of NUZYRA during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown) and enamel hypoplasia.
The use of NUZYRA during the second and third trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth.
Hypersensitivity reactions have been reported with NUZYRA. Life-threatening hypersensitivity (anaphylactic) reactions have been reported with other tetracycline-class antibacterial drugs. NUZYRA is structurally similar to other tetracycline-class antibacterial drugs and is contraindicated in patients with known hypersensitivity to tetracycline-class antibacterial drugs. Discontinue NUZYRA if an allergic reaction occurs.
Clostridioides difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents and may range in severity from mild diarrhea to fatal colitis. Evaluate if diarrhea occurs.
NUZYRA is structurally similar to tetracycline-class antibacterial drugs and may have similar adverse reactions. Adverse reactions, including photosensitivity, fixed drug eruption, pseudotumor cerebri, and anti-anabolic action (which has led to increased BUN, azotemia, acidosis, hyperphosphatemia, pancreatitis, and abnormal liver function tests), have been reported for other tetracycline-class antibacterial drugs, and may occur with NUZYRA. Discontinue NUZYRA if any of these adverse reactions are suspected.
Prescribing NUZYRA in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%) are nausea, vomiting, infusion site reactions, alanine aminotransferase increased, aspartate aminotransferase increased, gamma-glutamyl transferase increased, hypertension, headache, diarrhea, insomnia, and constipation.
DRUG INTERACTIONS
Patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage while taking NUZYRA.
Absorption of tetracyclines, including NUZYRA is impaired by antacids containing aluminum, calcium, or magnesium, bismuth subsalicylate and iron containing preparations.
USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding is not recommended during treatment with NUZYRA.
Please see Full Prescribing Information for NUZYRA.
References:
- Wrotslavsky P. Empiric treatment approach for the management of post-operative incisional infections with omadacycline. FASTRAC. 2023;3(1):1-6.
- NUZYRA [Prescribing Information]. Paratek Pharmaceuticals, Inc.
- Data on File. Paratek Pharmaceuticals, Inc